
PAD Series Filter Cartridges
PAD Series Filter Cartridge is constructed of highly asymmetric polyethersulfone membrane from Germany and imported support layer...
PAD Series Filter Cartridge is constructed of highly asymmetric polyethersulfone membrane from Germany and imported support layer. Unique double layer hydrophilic polyethersul-fone contributes to its excellent filtration performance and reliable bacteria-intercepting ability. It is especially used in pharmaceutical industry with stringent requirement. All components of PAD filter cartridges comply with FDA regula-tions. This filter can withstand repeated steam sterilization.
Features
- Unique double layer hydrophilic polyethersulfone with double security makes it have reliable bacteria-intercepting ability, increasing filtration safety factor by more than 10 times.
- Large effective filtration area makes the filter longer service life and lower cost.
- Broad chemical compatibility (PH1-14), it is suitable for various pharmaceutical filtration.
- Structure Stabilization, it can withstand sterilization cycle with 50 times
- 100% integrity test ensures absolute sterilization
- Low protein adsorption
- ISO9001:2015 certified Quality Management System
- Full traceability to each filter with unique serial number
Applications
- Biological vaccine/blood products sterilization filtration
- API sterilization filtration
- Large infusion (LVP), small injection (SVP) sterilization filtration
- Ophthalmic preparation sterilization filtration
- Buffers and reagents sterilization filtration
| Dimension | |
| Outer Diameter | 69mm(2.72") |
| Length | 5”, 10”, 20”, 30”, 40” |
| Material of Constructions | |
| Media | PES |
| Support | PP |
| Cage/Core/End cap | PP |
| Connection Adaptor | SS Insert, PSU Insert |
| Seal Material Options | Silicone, EPDM, NBR, FKM, E-FKM |
| Operating Conditions | |
| Max. Operating Temperature | 80℃(176℉) |
| Max. Operating DP | 4 bar(58psi)@20℃(68℉),2 bar (29 psi)@80℃(176℉) |
| Sterilization | |
| Autoclave Sterilization | 125℃(257℉), 30min, 30cycles |
| SIP | 125℃(257℉), 30min, 30cycles |
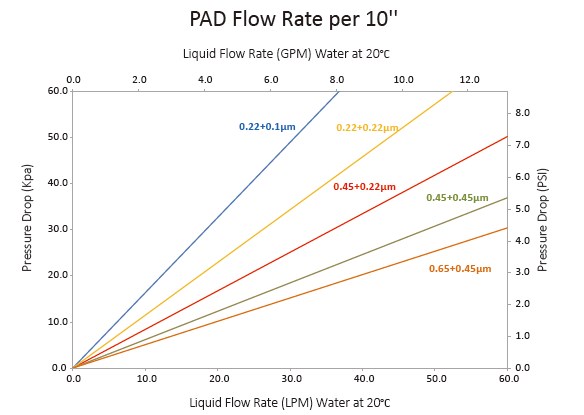
| Filtration Area | 0.65 m² per 10” Filter |
| Extractables | |
| 10” Filter Cartridges | <20mg |
Quality
- Validated with Brevundimonas diminuta (ATCC 19146) at107/cm2 (0.22μm).
- 100% Integrity Tested.
- Individual element is tracked by serial number.
- Manufactured according to ISO 9001:2015 certified quality management system.
- Meets USP Biological Reactivity Test requirements of the current USP <88> for plastic class Ⅵ-121℃.
Effluent quality
- Non-fiber releasing
- Non-pyrogenic per USP Bacterial Endotoxins(<0.25EU/mL)
- Meets TOC and water conductivity per USP Purified Water, pH per USP Sterile Purified Water.
Integrity Test

Order Information

catalog
Recommended Products
Recently Viewed Products
×
Download the documents after filling the form
- *Full Name:
- *Company:
- *Country:
- *Email Address:
- *Phone#:



